Jul 3 |
Nitazoxanide for COVID-19: real-time meta analysis of 14 studies | |
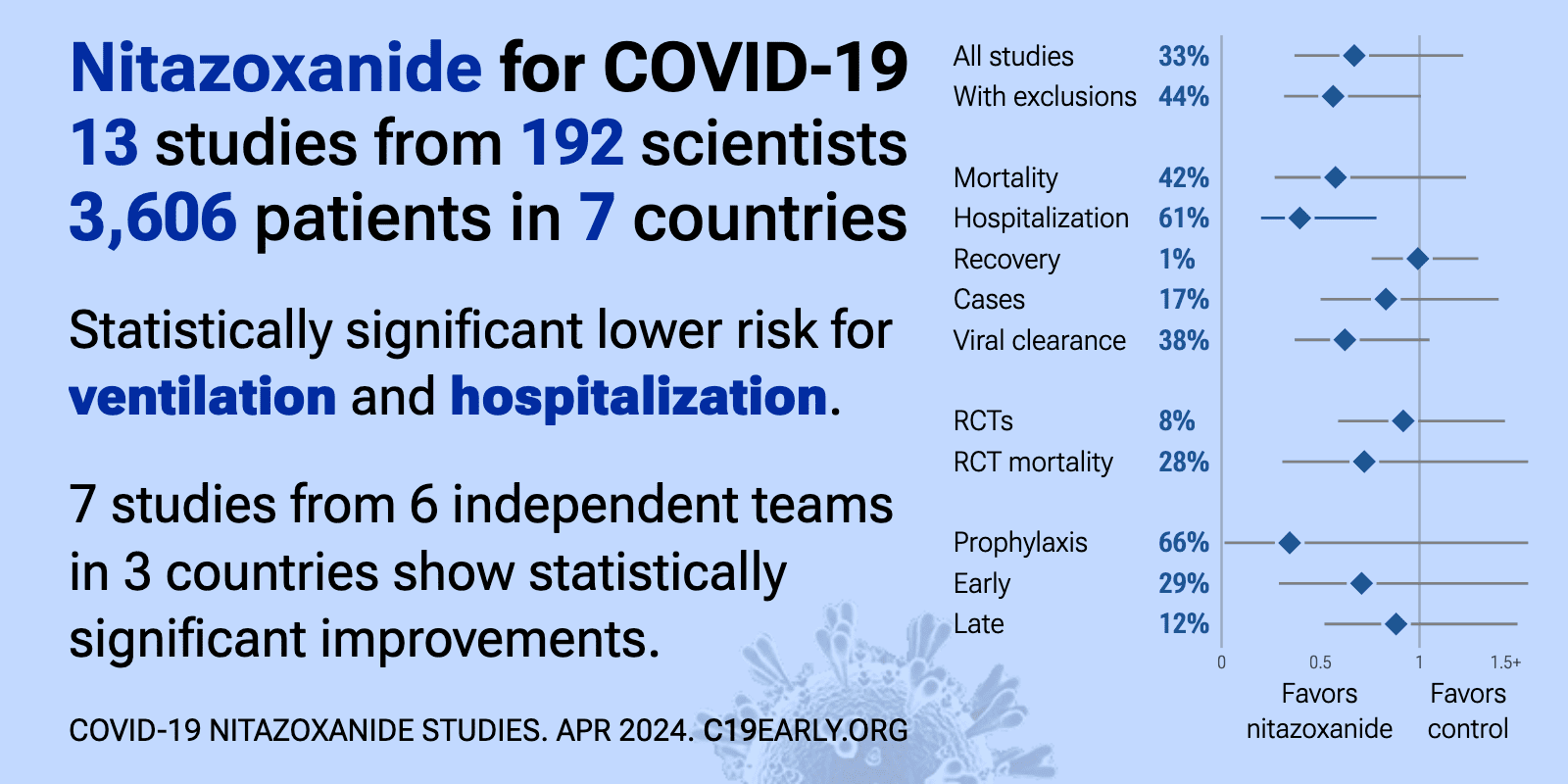
| Significantly lower risk is seen for ventilation and hospitalization. 8 studies from 7 independent teams in 4 countries show significant benefit. Meta analysis using the most serious outcome reported shows 35% [-8‑61%] lower ri.. | ||
Jun 26 2024 |
, NCT04359680 | Trial to Evaluate the Efficacy and Safety of Nitazoxanide (NTZ) for Pre- and Post Exposure Prophylaxis of COVID-19 and Other Viral Respiratory Illnesses (VRI) in Healthcare Workers and Others at Increased Risk of SARS-CoV-2 Infection |
| 43% lower progression (p=0.02), 50% faster recovery (p=0.1), and 3% fewer cases (p=1). RCT 1,407 healthcare workers and others at high risk of SARS-CoV-2 exposure, showing no difference in COVID-19 cases (13 in each group). There was lower symptom severity for nitazoxanide and a trend towards shorter illness duration. There.. | ||
Apr 30 2024 |
et al., Medical Research Archives, doi:10.18103/mra.v12i4.5252 | Nitazoxanide in the Treatment of COVID-19: A paradigm for Antiviral Drugs Targeting Host-Infected Cells |
| Review of nitazoxanide as a potential treatment for COVID-19. Authors highlight nitazoxanide's unique mechanism of action, which involves inhibiting mitochondrial oxidative phosphorylation in host cells, thereby lowering cellular ATP cont.. | ||
Apr 12 2024 |
et al., Global Journal of Aging & Geriatric Research, doi:10.33552/GJAGR.2024.03.000557 | Early Treatment Outcomes of SARS-Cov-2 with Ivermectin, Nitazoxanide and Acetylsalicylic Acid in 2 Nursing Homes During The COVID-19 Pandemic in Cali, Colombia |
| Retrospective 475 nursing home residents showing low mortality with early treatment using ivermectin, nitazoxanide, and acetylsalicylic acid. All residents were treated when the first positive cases were identified. 87 residents tested po.. | ||
Apr 3 2024 |
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23 | Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents |
| In Silico study supporting the synergistic combination of nitazoxanide and remdesivir for SARS-CoV-2. Authors developed a two-way pharmacodynamic modeling approach to capture the concentration-dependent drug-drug interactions and combined.. | ||
Mar 21 2023 |
et al., NCT04918927 | Favipiravir and/or Nitazoxanide: a Randomized, Double-blind, Placebo-controlled Trial of Early Antiviral Therapy in COVID-19 (FANTAZE) |
| 120 patient nitazoxanide early treatment RCT with results not reported over 2 years after completion. The protocol has been published [trialsjournal.biomedcentral.com]. | ||
Nov 3 2022 |
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2022.2142117 | Clinical outcomes, virological efficacy and safety of nitazoxanide in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials |
| Meta analysis of 5 RCTs showing improved viral clearance with nitazoxanide. Mortality was lower, but without statistical significance. | ||
Nov 1 2022 |
et al., eBioMedicine, doi:10.1016/j.ebiom.2022.104322 | Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial |
| 13% higher progression (p=0.89), 23% slower recovery (p=0.42), and 67% worse viral clearance (p=0.13). Very high COI low-risk patient RCT in South Africa, showing no significant differences with favipiravir plus nitazoxanide. There were no deaths and no COVID-19 hospitalizations for favipiravir plus nitazoxanide. More patients were seropos.. | ||
Oct 31 2022 |
et al., Clinical Drug Investigation, doi:10.1007/s40261-022-01213-y | The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials |
| Systematic review and meta analysis of 6 RCTs, showing significantly improved viral clearance and lower oxygen requirements with nitazoxanide, but no significant differences for mortality, ICU admission, and recovery. | ||
Aug 12 2022 |
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac266 | Randomized clinical trial of nitazoxanide or sofosbuvir/daclatasvir for the prevention of SARS-CoV-2 infection |
| 66% lower mortality (p=1), 79% lower hospitalization (p=0.5), 17% fewer symptomatic cases (p=0.49), and 21% more cases (p=0.67). Prophylaxis RCT 828 high-risk participants in South Africa, showing no significant difference with nitazoxanide and sofosbuvir/daclatasvir treatment. FLU-PRO results were available for 74% of the nitazoxanide arm compared to 54% of the co.. | ||
May 6 2022 |
et al., Arab Journal of Gastroenterology, doi:10.1016/j.ajg.2022.04.005 | Sofosbuvir/Ledipasvir in Combination or Nitazoxanide Alone are Safe and Efficient Treatments for COVID-19 Infection: A Randomized Controlled Trial for Repurposing antivirals |
| 56% improved viral clearance (p=0.02). RCT with 77 nitazoxanide, 70 sofosbuvir/ledipasvir, and 73 SOC patients in Egypt, showing faster viral clearance with nitazoxanide and with sofosbuvir/ledipasvir. There was no mortality or progression to severe COVID-19 or ICU admission. .. | ||
Apr 13 2022 |
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.844728 | Nitazoxanide in Patients Hospitalized With COVID-19 Pneumonia: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial |
| 5% higher mortality (p=0.95), 31% lower ICU admission (p=0.18), 40% lower need for oxygen therapy (p=0.06), and 64% faster improvement (p<0.0001). RCT late stage patients with COVID-19 pneumonia, 202 treated with nitazoxanide and 203 placebo patients, showing improved recovery, but no significant difference in mortality. | ||
Apr 7 2022 |
et al., Cellular and Molecular Life Sciences, doi:10.1007/s00018-022-04246-w | Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by nitazoxanide: an effect independent of spike variants emergence |
| In Vitro study showing that the host-directed broad-spectrum antiviral drug nitazoxanide may be effective for COVID-19 by hampering spike protein maturation and fusion activity. Authors note efficacy across alpha, beta, gamma and delta va.. | ||
Feb 28 2022 |
, News | ANTICOV Trial Finds Nitazoxanide/Ciclesonide Drug Combination Does Not Reduce Hospitalisation Risk in Patients With Mild COVID-19 |
| 188% higher progression (p=0.04). RCT with 462 nitazoxanide/ciclesonide and 443 paracetamol patients, up to 7 days from onset, showing no significant difference in progression. Minimal details, with the primary mortality outcome and treatment delay not being reported. | ||
Feb 9 2022 |
et al., bioRxiv, doi:10.1101/2022.02.08.479634 | The oral drug nitazoxanide restricts SARS-CoV-2 infection and attenuates disease pathogenesis in Syrian hamsters |
| Syrian hamster study showing improvements in SARS-CoV-2 related weight loss, inflammation, viral load, and lung synctia formation with nitazoxanide, and an In Vitro study showing that nitazoxanide inhibits SARS-CoV-2 in H9, iAT2, Vero E6,.. | ||
Feb 4 2022 |
et al., medRxiv, doi:10.1101/2022.02.03.22270152 | Efficacy and safety of nitazoxanide combined with ritonavir-boosted atazanavir for the treatment of mild to moderate COVID-19 |
| 11% worse recovery (p=0.73) and 5% improved viral clearance (p=0.92). Small RCT in Nigeria with 31 nitazoxanide and atazanavir/ritonavir patients, and 26 control patients, showing no significant differences with treatment. 4 treatment group patients discontinued treatment due to the size of the tablets. Tim.. | ||
Nov 23 2021 |
et al., PAMJ - Clinical Medicine, doi:10.11604/pamj-cm.2021.7.15.30981 | Treatment with hydroxychloroquine vs nitazoxanide in patients with COVID-19: brief report |
| 68% lower mortality (p=0.38), 87% lower ventilation (p=0.15), 59% lower ICU admission (p<0.0001), and 52% shorter hospitalization (p=0.007). Planned RCT of HCQ vs. HCQ+nitazoxanide which was aborted due to the retracted Surgisphere paper. Authors retrospectively analyze a small set of HCQ vs. nitazoxanide patients (which were protocol deviations in the planned RCT), showing re.. | ||
Sep 11 2021 |
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2463 (date from preprint) | An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2 |
| Phase I trial of high dose nitazoxanide, 1500mg twice daily, with 14 participants. Treatment was safe and well tolerated. PBPK predictions were confirmed on day 1 but with underprediction at day 5. Median Cmin was above the in vitro targe.. | ||
Aug 10 2021 |
et al., News Report | Mantiene Hospital Mónica Pretelini bajo índice de muertes Covid de mujeres embarazadas |
| News report on the use of nitazoxanide for pregnant COVID-19 patients in a clinic in Mexico, reporting significant improvements in hospitalization and mortality compared to locations without treatment. | ||
May 13 2021 |
et al., Cureus, doi:10.7759/cureus.15002 | Evolution of COVID-19 Pregnancies Treated With Nitazoxanide in a Third-Level Hospital |
| Case study of 51 COVID-19 positive pregnant women in Mexico treated with nitazoxanide, showing much better survival compared to hospitals not using nitazoxanide. | ||
Apr 20 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101310 (date from preprint) | A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19 |
| 79% lower hospitalization (p=0.22), 85% lower severe cases (p=0.07), and 7% slower recovery (p=0.88). RCT with 184 outpatients treated with an extended release formulation of nitazoxanide, and 195 controls, showing lower hospitalization and progression to severe disease with treatment. There was one COVID-19 related death in the treatment.. | ||
Mar 5 2021 |
et al., Medical Research Archives, doi:10.18103/mra.v11i2.3364 (date from preprint) | Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study |
| 26% improved viral clearance (p=0.36). Small RCT with 23 nitazoxanide and 13 control patients showing significantly more patients achieved over 35% reduction in viral load from baseline. NCT04463264. | ||
Feb 28 2021 |
et al., Molecular Therapy, doi:10.1016/j.ymthe.2020.12.016 | Synergistic and Antagonistic Drug Combinations against SARS-CoV-2 |
| In SIlico and In Vitro study showing both synergistic and antagonistic drug combinations against SARS-CoV-2 in Vero E6 cells. Authors screened 73 combinations of 32 drugs and identified 16 synergistic and 8 antagonistic combinations. Nita.. | ||
Feb 16 2021 |
et al., Journal of Medical Virology, doi:10.1002/jmv.26880 | Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-1 |
| 87% improved viral clearance (p<0.0001). Non-randomized controlled trial with 62 mild and early moderate patients with home treatment with ivermectin + nitazoxanide + ribavirin + zinc, showing significantly faster viral clearance. | ||
Jan 22 2021 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100981 (date from preprint) | Nitazoxanide superiority to placebo to treat moderate COVID-19 – A Pilot prove of concept randomized double-blind clinical trial |
| 67% lower mortality (p=0.25), 62% lower ventilation (p=0.17), 20% lower severe cases, and 56% shorter hospitalization (p=0.02). RCT with 25 nitazoxanide patients and 25 control patients, showing improved virological and clinical outcomes with treatment. Authors also perform an in vitro study in Vero E6 cells showing 90% inhibition with 0.5µM, with no cytotoxicity... | ||
Dec 31 2020 |
et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619 | Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis |
| In Silico physiologically based pharmacokinetic (PBPK) modeling study predicting optimal doses of nitazoxanide to maintain plasma and lung concentrations of the active metabolite tizoxanide above the SARS-CoV-2 EC90 in >90% of patients. A.. | ||
Nov 4 2020 |
et al., New Microbes and New Infections, doi:10.1016/j.nmni.2021.100915 (date from preprint) | Early COVID-19 Therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in Outpatient Settings Significantly Improved COVID-19 outcomes compared to Known outcomes in untreated patients |
| 88% lower mortality (p=0.08), 97% lower ventilation (p<0.0001), and 99% lower hospitalization (p<0.0001). Comparison of HCQ, nitazoxanide, and ivermectin showing similar effectiveness for overall clinical outcomes in COVID-19 when used before seven days of symptoms, and overwhelmingly superior compared to the untreated COVID-19 population, ev.. | ||
Oct 23 2020 |
et al., European Respiratory Journal, doi:10.1183/13993003.03725-2020 (date from preprint) | Early use of nitazoxanide in mild Covid-19 disease: randomized, placebo-controlled trial (preprint 10/23) |
| 404% higher ICU admission (p=0.24), 2% higher hospitalization (p=1), 16% worse recovery (p=0.37), and 12% improved viral clearance (p=0.006). RCT 392 patients, median treatment delay 5 days, showing improved viral recovery at 5 days. Symptom recovery was no different at 5 days, and the treatment arm had two ICU admissions compared to zero for control. There were no serious adve.. | ||
May 20 2020 |
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1909 | Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics |
| Pharmacokinetic analysis predicting that ivermectin, HCQ, CQ, and azithromycin will achieve lung concentrations well over 10 times higher than the reported EC50. Nitazoxanide had a lung tissue Cmax/EC50 of 7.8. | ||
Apr 30 2020 |
et al., Journal of Virus Eradication, doi:10.1016/S2055-6640(20)30017-0 | Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19 |
| Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. Nine RCTs comparing nitazoxanide with placebo or active control for 5-14 days in participants experiencing acute infections were included, accountin.. | ||
Please send us corrections, updates, or comments.
c19early involves the extraction of 200,000+ datapoints from
thousands of papers. Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation. IMA and WCH
provide treatment protocols.
Thanks for your feedback! Please search before submitting papers and note
that studies are listed under the date they were first available, which may be
the date of an earlier preprint.